Inside the Laboratory
As a ISO/IEC 17043:2023 accredited service, our specimen production is carried out by experienced scientists and backed by expert EQA Programme design.
Accredited to ISO/IEC 17043:2023
Participation is open to all clinical / research laboratories and point of care providers in the UK and overseas
Part of the NHS, is a non-profit Proficiency Testing provider accredited by UKAS to ISO/IEC 17043:2023 (provider no. 8560) working within NHS Greater Glasgow and Clyde.
Building confidence with clinically relevant levels of cardiac biomarkers, robust analysis of results by experienced scientists using well established procedures to produce state-of-the-art EQA reports.
Organised with a formal Steering Committee and membership of the UK NEQAS Specialist Advisory Group for Immunoassay.
Routine educational and participant meetings as well as annual questionnaires to solicit participant feedback.
Our EQA programme forms part of the official repertoire of UK NEQAS offerings. The troponin EQA programme has been in operation since 1998.
Cardiac markers, especially Troponin I (cTnI) and Troponin T (cTnT), are critical measurements in acute coronary syndrome. Most authorities recommend that these measurements are carefully quality controlled.
Firstly may I congratulate you on an excellent CMD dialogues meeting. It was very informative.
As a ISO/IEC 17043:2023 accredited service, our specimen production is carried out by experienced scientists and backed by expert EQA Programme design.
Combined samples are issued for troponin, CKMB, myoglobin and NT-proBNP. An additional sample for troponin analysis only is issued for high sensitivity methods to cover very low clinically important levels around the assay detection limit.
Join a UK NEQAS Cardiac Markers EQA Programme.
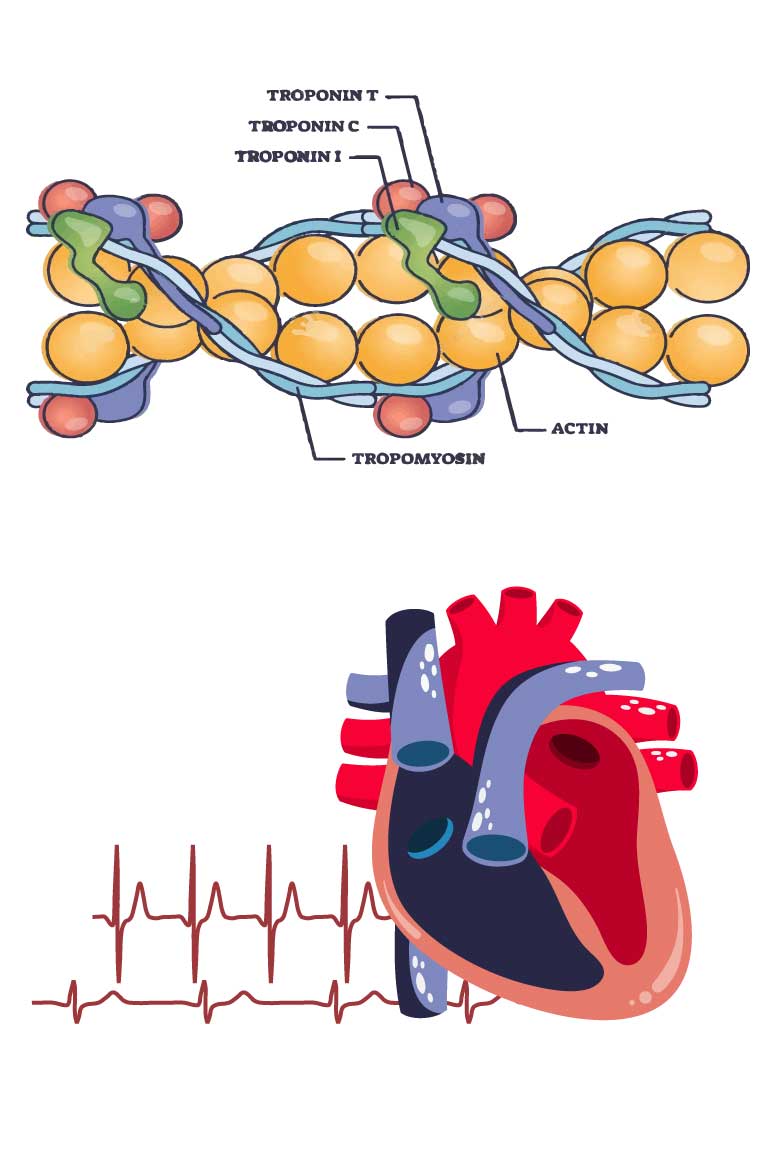
UK NEQAS Cardiac Markers is organised to provide a UK NEQAS EQA Programme for the assay of cardiac Troponin I and Troponin T (including high sensitivity methods), Creatine kinase-MB, Myoglobin, B-type Natriuretic Peptide and N-Terminal -ProB-type Natriuretic Peptide and will develop appropriate EQA Programmes for new cardiac biomarkers. EQA Programmes are available for both laboratory systems and for point of care systems

Point of care methods for Cardiac Troponin I, Cardiac Troponin T☨, CKMB☨, Myoglobin☨ and NT-proBNP☨.
☨ Analyte not currently accredited to ISO/IEC 17043:2023
Contact UsParticipation is open to all clinical / research laboratories and point of care providers in the UK and overseas.